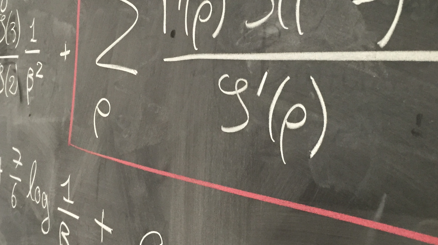Beatriz Farah
Sample size calculation based on differences of quantiles from right-censored data
When evaluating treatment effect, it is common to rely on the hazard ratio, typically by using the ubiquitous Cox model. In the presence of right-censoring, the hazard rate can be easily estimated from the observed data which makes this model very appealing. In Randomized Clinical Trials (RCT), standard methods already exist to determine the sample size when the estimand is a hazard ratio (typically the hazard ratio for comparing the effect of two treatments) based on either the log-rank test or the Cox model. However, in cancer studies, some treatments may have a late effect and the proportional hazard assumption imposed by the Cox model is no longer verified. Thus, we would like to shift from the hazard ratio to the difference in quantile of failure time as estimand because:
1) It allows for quantifying different treatment effects across quantiles;
2) Quantile regression doesn’t assume proportional risks, making it appropriate for
analyzing delayed treatment effects associated with immunotherapy;
3) It offers a clinically interpretable way to measure the benefit of one treatment over another as a function of time.
Our goal is to propose a sample size formula for evaluating treatment effects by comparing pre-specified quantiles in each treatment group.
A promising method for testing equality of quantiles was proposed by Kosorok (1999), which allows to either test simultaneously different quantiles or to test the same quantile at different analysis times in a group sequential clinical design. This method needs an estimator of the density of the distributions at the quantiles, for which we propose a gaussian resampling method inspired by Lin et. al. (2015).
We propose an explicit expression for the power of the test which allows us to derive a formula for computing minimal sample size. Simulation studies are performed to show that the proposed approach provides reasonable type I probabilities and powers. The described procedure is also applied to data from phase III non-small-cell lung cancer clinical trials.