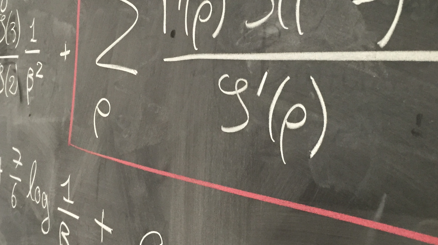Léa Comin (CEA – HeKA)
Modelling the whole-body pharmacokinetics of radiolabeled glyburide in healthy volunteers
Microdosing approaches allow the administration of infra-therapeutic doses of drug candidates to humans. Decisive clinical PK can therefore be obtained at a early stage compared to the traditional process. A microdose PET study was performed in subjects to assess the role of Organic Anion-Transporting Polypeptides (OATP) for liver uptake and their impact on whole-body pharmacokinetics (WBPK), using the substrate Glyburide as a model drug.
The aim of our work was to model the WBPK of radiolabeled glyburide (11C-glyburide) with or without pre-infusion of rifampicin, a potent inhibitor of OATPs. We used Nonlinear Mixed Effect models (NLMEM), consisting of compartmental and statistical models. Intra- and inter-individual variability were studied, and correlations and covariates were incorporated into the model. Finally, we compared our estimated parameters with those using blood samples in literature, the traditional process.